What Is The Role Of An Electrode . electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface,. In batteries and fuel cells, electrodes play a crucial role in storing and converting energy. some common classifications include: [1] electrodes are commonly used in electrochemical cells (see. electrodes are substances that are usually used to make electrical contact with a nonmetallic part. The word electrode comes from two greek. an electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. to help you understand the concept in simple terms, an electrode is a point where the current enters and leaves the electrolyte. electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting.
from www.doubtnut.com
[1] electrodes are commonly used in electrochemical cells (see. In batteries and fuel cells, electrodes play a crucial role in storing and converting energy. to help you understand the concept in simple terms, an electrode is a point where the current enters and leaves the electrolyte. The word electrode comes from two greek. an electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. electrodes are substances that are usually used to make electrical contact with a nonmetallic part. electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting. electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface,. some common classifications include:
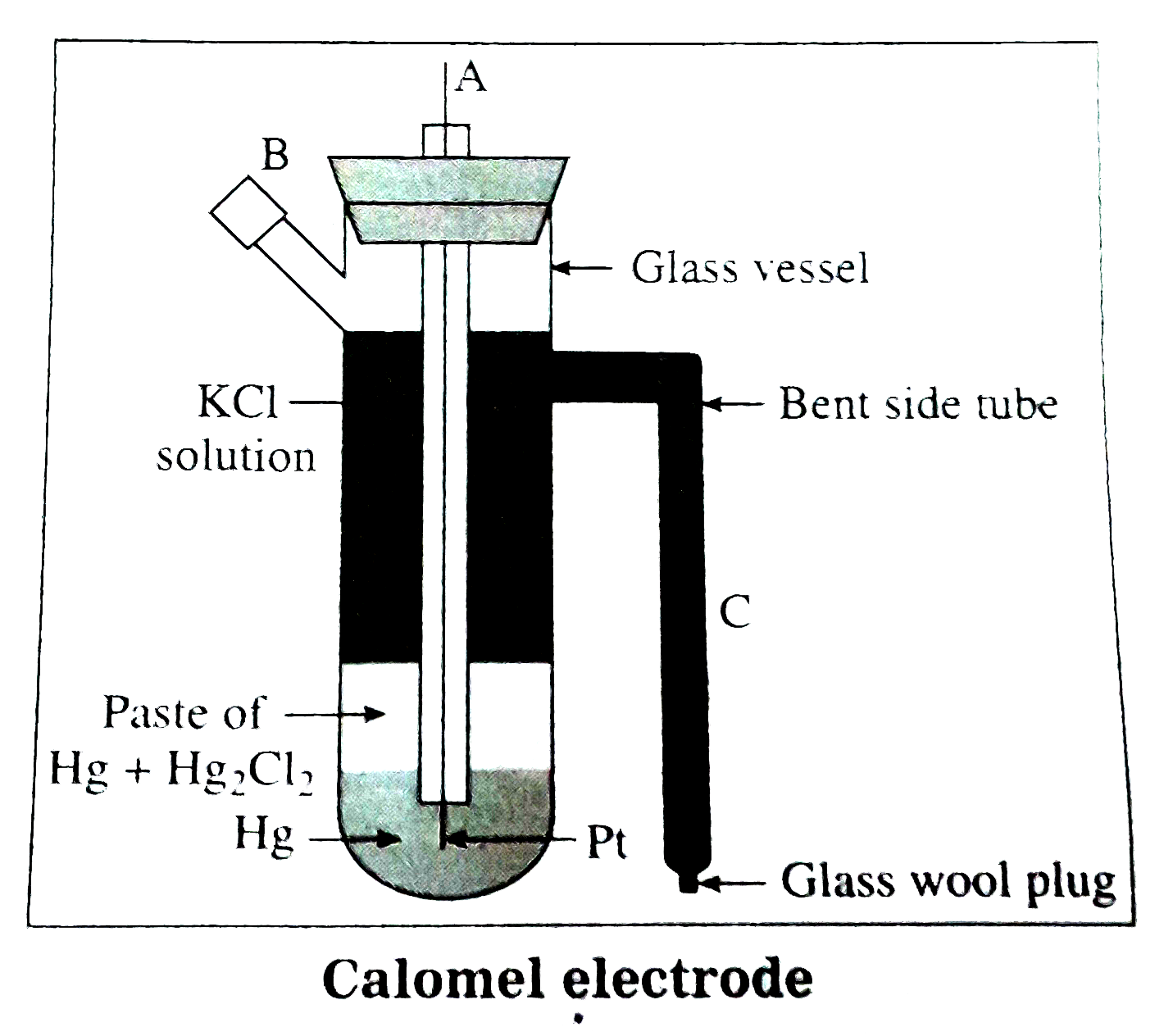
Describe the construction and working of the calomel electrode.
What Is The Role Of An Electrode an electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. In batteries and fuel cells, electrodes play a crucial role in storing and converting energy. some common classifications include: The word electrode comes from two greek. electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface,. to help you understand the concept in simple terms, an electrode is a point where the current enters and leaves the electrolyte. an electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. [1] electrodes are commonly used in electrochemical cells (see. electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting. electrodes are substances that are usually used to make electrical contact with a nonmetallic part.
From byjus.com
What is an active electrode? Explain with the help of an example What Is The Role Of An Electrode In batteries and fuel cells, electrodes play a crucial role in storing and converting energy. electrodes are substances that are usually used to make electrical contact with a nonmetallic part. [1] electrodes are commonly used in electrochemical cells (see. an electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. . What Is The Role Of An Electrode.
From chem.libretexts.org
1.7 Ion Selective Electrode Analysis Chemistry LibreTexts What Is The Role Of An Electrode In batteries and fuel cells, electrodes play a crucial role in storing and converting energy. some common classifications include: electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface,. electrodes are substances that are usually used to make electrical contact with a nonmetallic part. The word electrode comes. What Is The Role Of An Electrode.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types What Is The Role Of An Electrode electrodes are substances that are usually used to make electrical contact with a nonmetallic part. [1] electrodes are commonly used in electrochemical cells (see. electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface,. In batteries and fuel cells, electrodes play a crucial role in storing and converting energy.. What Is The Role Of An Electrode.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry What Is The Role Of An Electrode The word electrode comes from two greek. an electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. [1] electrodes are commonly used in electrochemical cells (see. In batteries and fuel cells, electrodes play a crucial role in storing and converting energy. electrode, electric conductor, usually metal, used as either of. What Is The Role Of An Electrode.
From www.youtube.com
Explain the origin of single electrode potential? Electrochemistry What Is The Role Of An Electrode [1] electrodes are commonly used in electrochemical cells (see. electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting. electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface,. In batteries and fuel cells, electrodes play a crucial role in storing and converting. What Is The Role Of An Electrode.
From www.doubtnut.com
Describe the construction and working of the calomel electrode. What Is The Role Of An Electrode In batteries and fuel cells, electrodes play a crucial role in storing and converting energy. [1] electrodes are commonly used in electrochemical cells (see. The word electrode comes from two greek. to help you understand the concept in simple terms, an electrode is a point where the current enters and leaves the electrolyte. electrode, electric conductor, usually metal,. What Is The Role Of An Electrode.
From cexpdriy.blob.core.windows.net
What Is The Purpose Of An Electrode at James Davis blog What Is The Role Of An Electrode The word electrode comes from two greek. an electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. some common classifications include: In batteries and fuel cells, electrodes play a crucial role in storing and converting energy. electrode reactions are a class of chemical reactions that involve the transfer of. What Is The Role Of An Electrode.
From stock.adobe.com
Electrolytic cell infographic diagram with components including anode What Is The Role Of An Electrode some common classifications include: [1] electrodes are commonly used in electrochemical cells (see. an electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. The word electrode comes from two greek. In batteries and fuel cells, electrodes play a crucial role in storing and converting energy. electrodes are substances that. What Is The Role Of An Electrode.
From www.slideserve.com
PPT The Role of Electrode Material in Applied Electrochemistry What Is The Role Of An Electrode The word electrode comes from two greek. [1] electrodes are commonly used in electrochemical cells (see. an electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. electrodes are substances that are usually used to make electrical contact with a nonmetallic part. electrode reactions are a class of chemical reactions. What Is The Role Of An Electrode.
From blog.usesi.com
What is an Electrode? USESI What Is The Role Of An Electrode The word electrode comes from two greek. to help you understand the concept in simple terms, an electrode is a point where the current enters and leaves the electrolyte. electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting. electrodes are substances that are usually used to make electrical contact with. What Is The Role Of An Electrode.
From www.researchgate.net
5 (a) Glass electrode (b) Combined electrode Download Scientific Diagram What Is The Role Of An Electrode electrodes are substances that are usually used to make electrical contact with a nonmetallic part. In batteries and fuel cells, electrodes play a crucial role in storing and converting energy. The word electrode comes from two greek. electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface,. electrode,. What Is The Role Of An Electrode.
From 35.82.204.13
ECG Electrode Placement Open Critical Care What Is The Role Of An Electrode electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface,. The word electrode comes from two greek. electrodes are substances that are usually used to make electrical contact with a nonmetallic part. an electrode is a conductor that is used to make contact with a nonmetallic part of. What Is The Role Of An Electrode.
From www.researchgate.net
Illustration of a small part of an electrode, including active What Is The Role Of An Electrode The word electrode comes from two greek. an electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. [1] electrodes are commonly used in electrochemical cells (see. electrodes are substances that are usually used to make electrical contact with a nonmetallic part. electrode reactions are a class of chemical reactions. What Is The Role Of An Electrode.
From studycopesettic.z21.web.core.windows.net
Inert Electrodes Gcse What Is The Role Of An Electrode to help you understand the concept in simple terms, an electrode is a point where the current enters and leaves the electrolyte. an electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. The word electrode comes from two greek. In batteries and fuel cells, electrodes play a crucial role in. What Is The Role Of An Electrode.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry What Is The Role Of An Electrode an electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface,. electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting. The word electrode comes. What Is The Role Of An Electrode.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision What Is The Role Of An Electrode In batteries and fuel cells, electrodes play a crucial role in storing and converting energy. to help you understand the concept in simple terms, an electrode is a point where the current enters and leaves the electrolyte. [1] electrodes are commonly used in electrochemical cells (see. some common classifications include: electrodes are substances that are usually used. What Is The Role Of An Electrode.
From greenenergymaterial.com
What Is Electrode Potential And Their Applications » Green Energy Material What Is The Role Of An Electrode In batteries and fuel cells, electrodes play a crucial role in storing and converting energy. to help you understand the concept in simple terms, an electrode is a point where the current enters and leaves the electrolyte. electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface,. [1] electrodes. What Is The Role Of An Electrode.
From www.sciencenewsforstudents.org
Explainer What is an electrode? Science News for Students What Is The Role Of An Electrode [1] electrodes are commonly used in electrochemical cells (see. to help you understand the concept in simple terms, an electrode is a point where the current enters and leaves the electrolyte. In batteries and fuel cells, electrodes play a crucial role in storing and converting energy. some common classifications include: The word electrode comes from two greek. . What Is The Role Of An Electrode.